Lupine Publishers | Crystallization and Polymorphism-Scalable Process for Celecoxib and It’s Polymorph From-3 (Non- Steroidal Anti-Inflammatory Drug (NSAID)
Abstract
The present process
provides an improved process for the preparation of 4-[5-(4-methylphenyl)-3-
(trifluoromethyl)-1Hpyrazol- 1-yl] benzene sulfonamide (Celecoxib) and its
purification and crystallization to produce polymorph. The present process,
which describes the manufacturing process of Celecoxib, which is a non-
steroidal anti-inflammatory drug (NSAID), has the advantage of scaling up to
the industrial level of production. The process uses safe reagents in the
process which makes it for industrial scale operations. The yields in the
process are high, which makes it a cost-effective process. Formation of isomers
are less compared with the all existing process, which makes it effective to
make it to the pharmacopoeia grade. Residual solvents play a very important
role in the impurity profile of APIs as per the ICH Guidelines ICH Q3C (R4). In
this process by carrying out the final step of condensation in the aqueous
medium followed by crystallization, the residual solvents limits are well taken
care of.
Keywords: Non-steroidal anti-inflammatory drug (NSAID); Celecoxib;
Cyclooxygenase 2; X-ray diffraction; Polymorphism; Process
Discussion
Figure 1: Classes of multi component molecular crystals.
Figure 1 present process
relates to “AN IMPROVED PROCESS FOR THE PREPARATION OF CELECOXIB POLYMORPH
FORM”. Celecoxib is designated chemically as 4-[5-(4-methylphenyl)-3-
(trifluoromethyl)- 1H-pyrazol-1-yl] benzene sulphonamide and is a
diaryl-substituted pyrazole [1]. The compound has the following structure
(Figure 2).
Figure 2: 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1Hpyrazol- 1-yl] benzene
sulfonamide.
The drug is currently
marketed as Celebrex® in the United States of America by Pharmacia Corporation.
Celecoxib is a non-steroidal anti-inflammatory drug (NSAID) [1] mainly used in
treatment of arthritis, pain, menstrual cramps, and colonic polyps. Celecoxib
blocks the enzyme (cyclooxygenase 2) which makes prostaglandins, resulting in
lowering the concentrations of prostaglandins. As a consequence, reduction in
inflammation and its accompanying pain, fever, swelling and tenderness. The
manufacture of Celecoxib has been described in various patents and to cite a
few references, G. D. Searl & Co. has disclosed method for preparation of
Celecoxib [2-3] in US 5,466,823 which is as under: US 5,134,142 [2], US
5,563,165, US 6,150,534, US 5,892,053, US 2007/0004924, US 2008/0234491, EP
1,528,058, EP 1,167,355, EP 2,246,332, WO 01/42221, WO 03/090730, WO05/014546,
WO 06/051340, WO 08/145733, and WO 2010/095024 have also described the
synthesis of Celecoxib Reddy et al in their publication in Org. Process Res.
Dev., 2009, 13(1), pp 98-101. have disclosed the synthesis (Figure 3).
Figure 3: Manufacture of Celecoxib.
Detailed Description of the Drawings
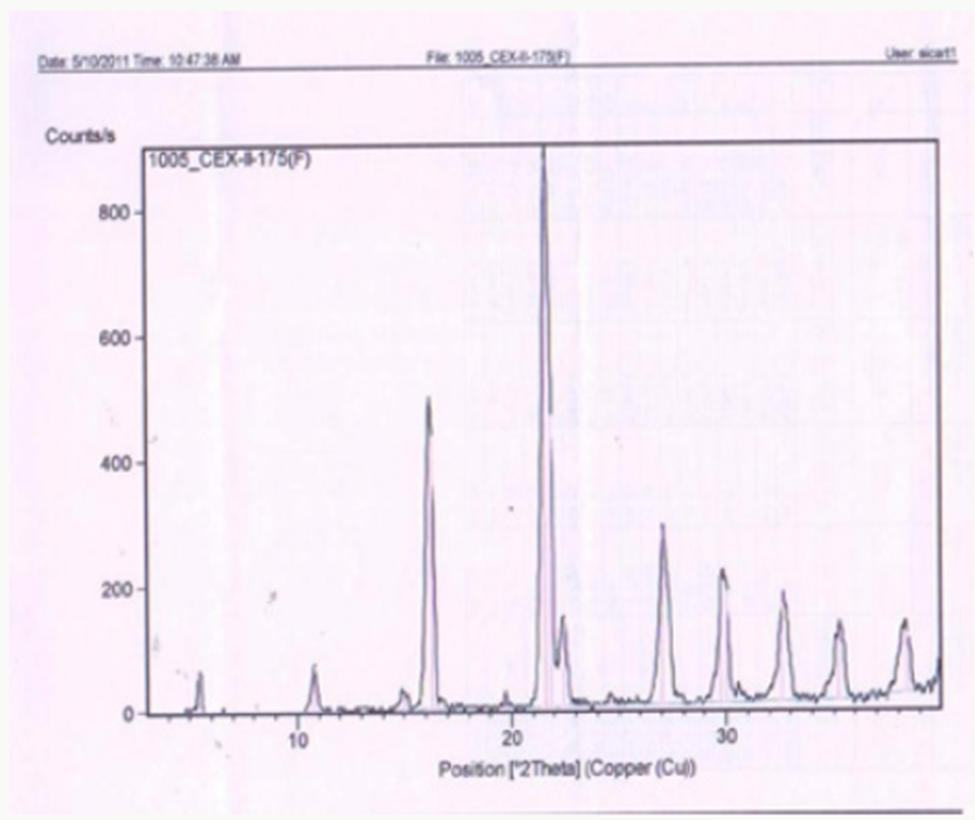
Figure 1 describes the
powder X-ray diffraction pattern of the Celecoxib Polymorph; Figure 2
illustrates 2θvalues. Figure 3 depicts the DSC thermogram taken at 10:C /min
over a temperature range of 30:C to 200:C for Celecoxib polymorphic form.
Description of the Process
Figure 4: Crystals of Celecoxib polymorph.
The present procedure
describes the preparation of Celecoxib by a novel process and its
crystallization to polymorphic form. The present process for the preparation of
Celecoxib by a process involving condensation of 4,4,4-trifluoro-1-[4-(methyl)
phenyl]- butane-1,3-dione [1] with sulphonamido phenyl hydrazine hydrochloride
[2] in an aqueous medium to give Celecoxib [3]. This is followed by
crystallization from a mixture of solvents [4- 8] containing Aromatic
hydrocarbon and aliphatic ketone. In the condensation reaction the reactants
are added in water and reactions done at ambient temperature. The crude
Celecoxib is isolated by filtration. In the for purification of Celecoxib and
its crystallization to polymorphic FORM Preparing a solution of Crude Celecoxib
in a solvent mixture comprising of an aliphatic ketone (Acetone) and an
aromatic hydrocarbon (Toluene)at reflux temperature followed by cooling
crystallization to give crystals of Celecoxib polymorph [8-12] (Figure 4).
Table 1:
In this process by
carrying out the final step of condensation in the aqueous medium followed by
crystallization, the residual solvents limits are well taken care of. The
yields in the process are higher compared to the prior art, which makes it a
cost-effective process. Formation of isomers are less compared with the prior
art, which makes it effective to make it to the pharmacopoeia grade. Residual
solvents play a very important role in the impurity profile of APIs as per the
ICH Guidelines ICH Q3C (R4). In this process by carrying out the final step of
condensation in the aqueous medium followed by crystallization, the residual
solvents limits are well taken care of [13,14]. The crystallization conditions
are well established to give crystalline polymorph. The powder X-Ray
diffraction pattern of the Celecoxib is given in Figure 1 and 2θ values are
given in Table 1 of Figure 2. The differential scanning calorimeter graph of
the Celecoxib polymorph under specific conditions shows the melting point
around 162.7˚C. The DSC of Celecoxib is given in Figures 3,5, and 6.
Figure 5: Solid Dosage Forms.
Figure 6: Process induced transformations.
Solid Forms
a) Propensity to produce
different forms not significantly different for salts and non-salts.
b) Need more data on
co-crystals (Figure 7).
The details of the new
methods for preparation of celecoxib are further illustrated in the following
examples.
Example 1: Preparation of Celecoxib
In a 20 liter 3-necked flask, equipped with stirrer, thermometer
and reflux condenser, deionized water (7.9 Liter) is charged and mixture of
4,4,4-trifluoro-1-[4-(methyl) phenyl]-butane-1,3-dione (1.6Kg; 6.95×103mmoles)
and 4-sulphonamido phenyl hydrazine hydrochloride (1.7Kg; 7.57×103mmoles), a resultant
mixture was heated at 75˚C to 80˚C and maintained for 5 hours. The reaction
mixture was cooled to 25˚C to 30˚C to give a slurry. The slurry was filtered
and washed with water (3.2liter) wet- cake was collected and further processed
for purification as given below.
Figure 7: Percentages of forms from Polymorph Screening.
a) Purification and Crystallization to Give Polymorph: Celecoxib wet-cake obtained in the process
described above was taken into 20 liter 3-necked flask, equipped with stirrer,
thermometer and reflux condenser, mixture of acetone (0.54liter) and toluene
(10.8liter) was added and the reaction mixture was heated to 80˚C to 85˚C for
30 minutes. Activated carbon (0.3Kg) was added and the reaction mixture was
further heated to 80˚C to 85˚C. The reaction mixture was cooled to 25˚C -30˚C.
The slurry was filtered, washed with toluene and then dried at 70˚C to yield the
Celecoxib polymorph compound1.35 kg (HPLC purity-99.8% & molar yield;
50.9%).
IR: 3340, 3240, 1600,
1500, 1350, 1280, 1235, 1160, 980, 910, 840, 800,760, 635, 560, 530 cm-1 (KBr pellet)
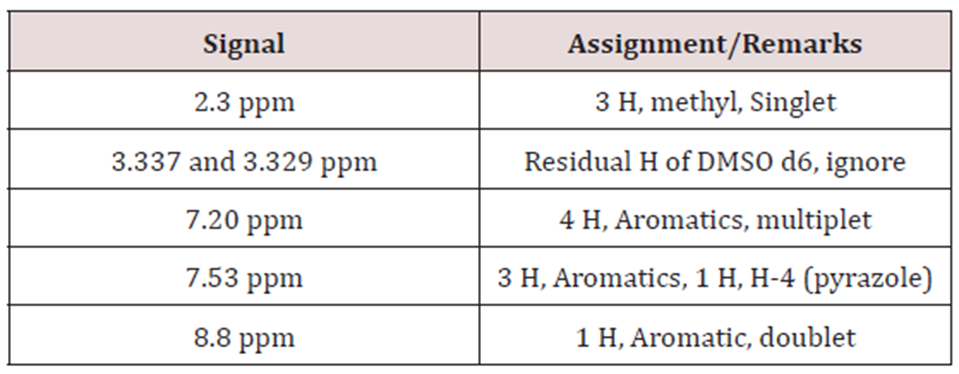
Proton NMR: Solvent:
DMSO d6, 300 MHz.
Example 2: Preparation of Celecoxib
In a 20liter 3-necked flask, equipped with stirrer, thermometer
and reflux condenser, charge deionized water(9Liter) and mixture of
4,4,4-trifluoro-1-[4-(methyl)phenyl]-butane-1,3-dione(1.6Kg; 6.95×103mmoles)
and 4-sulphonamido phenyl hydrazine hydrochloride(1.7Kg; 7.57×103mmoles), a resultant
mixture was heated at 90˚C to 100˚C and maintained for 5 hours. The reaction
mixture was cooled to 25˚C to 30˚C. The slurry was filtered and washed with
water (3.2liter) wet-cake was collected and further processed for purification
as given below.
Figure 8: Powder X-Ray diffraction pattern of the Celecoxib.
a) Purification and crystallization to give Polymorph: Celecoxib wet-cake obtained in the process
described above was taken into 20liter 3-necked flask, equipped with stirrer,
thermometer and reflux condenser, mixture of acetone (0.54liter) and toluene
(10.8liter) was added and the reaction mixture was heated to 80˚C to 85˚C for
30 minutes. Activated carbon (0.3Kg) was added and the reaction mixture was
further heated to 80˚C to 85˚C. The reaction mixture was cooled to 25˚C -30˚C.
The separated solid was filtered, washed with toluene and then dried at 70˚C to
yield the Celecoxib polymorph compound1.24 kg (HPLC purity-99.3% & molar
yield; 47%) (Figures 8-11c).
Figure 9: 2θ values.
Figure 10: DSC of Celecoxib.
Figure 11c:
Conclusion
The distinct advantage
of the present method of preparation over the prior art can be summarized as
per below:
The present process,
which describes the manufacturing process of Celecoxib, which is a non-
steroidal anti-inflammatory drug (NSAID), has the advantage of scaling up to
the industrial level of production. The process uses safe reagents in the
process which makes it for industrial scale operations. The present process
provides an improved process for the preparation of 4-[5-(4-methylphenyl)-3-
(trifluoromethyl)-1H-pyrazol-1- yl] benzene sulfonamide (Celecoxib) and its
purification and crystallization to produce polymorph. The yields in the
process are high compared to existing process which makes it a cost-effective
process. Formation of isomers are less compared with the prior art, which makes
it effective to make it to the pharmacopoeia grade. In this process by carrying
out the final step of condensation in the aqueous medium followed by
crystallization, the residual solvents limits are well taken care of. The
yields in the process are higher compared to the prior art, which makes it a
cost-effective process. Residual solvents play a very important role in the impurity
profile of APIs as per the ICH Guidelines ICH Q3C (R4).
Read More Lupine Publishers Medical Sciences Articles: https://lupine-publishers-medical-sciences.blogspot.com/











No comments:
Post a Comment